Rutherford's Nuclear Model of Atom
Rutherford's Nuclear Model of Atom: Overview
This topic covers concepts, such as, Drawbacks of Rutherford Model of Atom, Rutherford Experiment, Relation Between Impact Parameter and Scattering Angle & Distance of Closest Approach in Alpha Particle Scattering Experiments etc.
Important Questions on Rutherford's Nuclear Model of Atom
In Rutherford's scattering experiment, the number of particles scattered at is . The Number of particle scattered at will be-
In Rutherford's experiment, number of particles scattered at angle are per second,Number particles scattered per second at angle is
Which one of the following conclusions could not be derived from Rutherford's particle scattering experiment?
A proton and an alpha particle are accelerated in a field of the same potential difference. Then, the ration of the Brigle wavelengths associated with the moving material particles is:
The number of particles scattered per unit area at scattering angle varies inversely as-
Which one did Rutherford consider to be supported by the results of experiments in which Particles were scattered by gold foil?
Determine the ration of distance of closest approach of a photon and an alpha particle, incident on a thin gold foil, if they are accelerated through same potential difference
An alpha nucleus of energy bombards a heavy nucleus target of charge . Then the distance of closest approach for the alpha nucleus will be proportional to
In a Rutherford scattering experiment when a projectile of charge and Mass approached a target nucleus of charge and Mass , the distance of closed approach is . The energy of projectile is
An particle of energy of is scattered through by a fixed uranium nucleus. The distance closed approach is of the order of-
Explain briefly how the Rutherford atomic model is not able to explain stability of the atom.
According to the rutherford's model, entire positive charge is concentrated in the centre of the atom.
Geiger-Marsden experiment is related with the size of the-
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
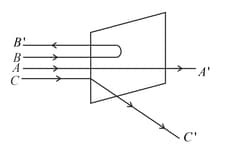
On which of the following factors does the trajectory of an alpha particle depend?
What will be the trajectory of an alpha particle if the impact parameter is large?
Calculate the impact parameter of a alpha particle scattered by when it approaches a gold nucleus ().
Rutherford proposed a nuclear model of the atom in which the atom is composed of a tiny nucleus at its edge containing positive charges.
An alpha particle of energy is scattered through by a fixed uranium nucleus. Then, the distance of closest approach is of the order of .
Explain the Rutherford's model of an atom.
